Do Adjuvants Need To Be Registered In Brazil
Unpredictability Rules Brazilian Pesticide Registrations
Challenges to inbound the most attractive and profitable pesticide market place in the world – estimated at $11.6 billion in 2013 – come as no surprise. Some of them are incommunicable to overcome. Indeed the pesticides registration system in Brazil is one of the most complex in the world. For starters, there are 3 federal agencies involved in registration, each with its own internal rules and procedures. They are: MAPA (Ministry of Agriculture); ANVISA (Ministry of Health) and IBAMA (Ministry of Environment), plus state registrations.
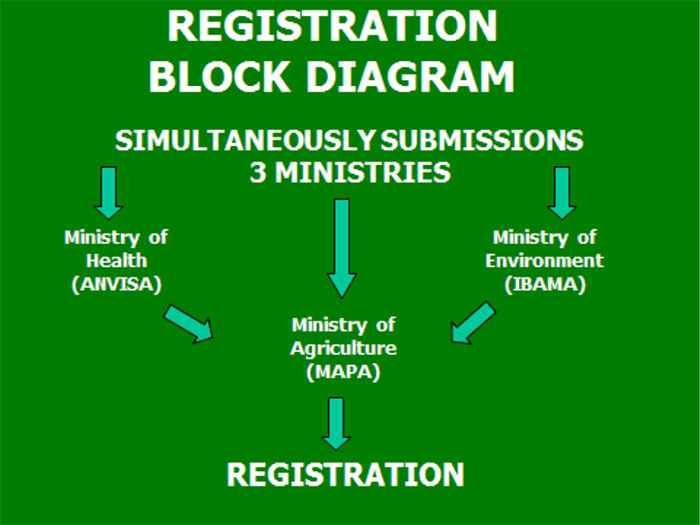
Since the new regulation apropos equivalency registration was approved at the cease of 2006, almost all major pesticide players in the earth including new local companies, started racing to apply for registrations to quickly access the marketplace.
Since then, approximately 250 formulations and 320 technical product registrations based on equivalency were canonical.
Until 2011, the evaluation of registrations took 2 to two.5 years to complete from the time of submission. Nonetheless later on internal investigations in ANVISA in 2012, when two managers were fired, the look time drastically increased. Not only did evaluations slow but the number of submissions also increased considerably adding to the delay.
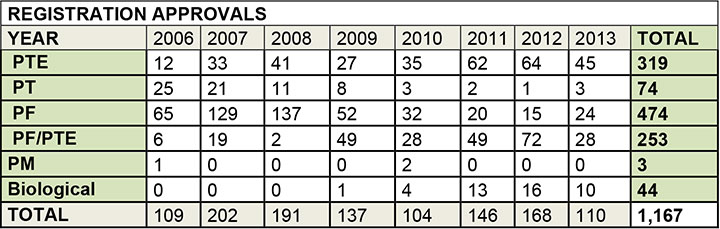
Source: Ministry of Agriculture, Jan. 2014
For today'south registration applications, co-ordinate to pessimistic estimates, the evaluation procedure will likely take from vii to 11 years. However it is reasonable to suggest that it could happen in as fiddling every bit 3 to 4 years from the time of awarding. Nonetheless there are some cases on record that were approved in less than 12 months.
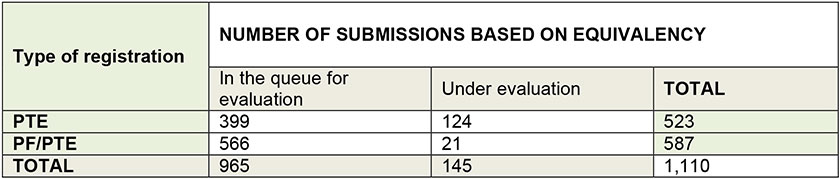
Source: ANVISA, Jan. 2014
Notes:
PTE: technical product based on equivalency
PT: technical product not based on equivalency
PF: formulation not based on equivalency
PF/PTE: formulation linked to a technical product registration based on equivalency
PM: pre-mixture
Uncertainties concerning regulations take dampened the confidence that the interested companies have in this market and have often caused them to postpone their plans for the futurity. The number of new players notwithstanding, registration submissions are definitely increasing.
Critical Issues
– Registration evaluation conclusions: unpredictability and delays.
– Unclear criteria for production registration and lack of standard processes.
– Recently regulatory authorities deliberated that on-going registration submissions cannot exist transferred and merchandise names cannot exist changed until blessing.
– ANVISA and IBAMA, two of the iii agencies involved in registration, consider that a product is not eligible for registration when ane is already registered that poses a more environmental/toxicological take a chance. This understanding was for several years only for new active ingredients.
– EUP (experimental apply permit): maximum catamenia of validity 6 years. Too short considering registration evaluations may take up to 4 to 5 years.
– EUP approvals with mistakes: corrections may take 2 to half-dozen months.
– Batch sample for toxicological testing (6 pack and 2 mutagenicity): should be performed with a called batch by ANVISA.
A projection to amend the registration regulatory Decree 4074 with the objective to speed up registration evaluations had been discussed in 2013 with agribusiness key players including regulatory agencies. Every bit expected, it was discontinued. Thus for the current twelvemonth, with the World Loving cup in Brazil and presidential elections soon subsequently, the outlook for change of the bodily state of affairs is discouraging.
Taking into account the unpredictability and delays on registration evaluation conclusions, some companies are considering the possibility of suing regulatory agencies. In fact, in that location are recorded cases in which registration applicants won lawsuits against agencies. Certainly the conclusion to sue a regime agency should be very well thought out since no visitor aims to counter the establishment.
Considering of a lack of manpower, regulatory agencies are restricted to deadlines. Thus, in the brusque term, the outlook is not very optimistic as the queue of registration submissions is ascent, while evaluations are non keeping pace.
Yet more and more players are willing to take risks in this very uncertain path to the biggest global pesticide market – where 26% of submissions for technical products are rejected. For those who overcome this barrier to entry, the war is non won still. Further, many other battles are however to be won. Just through good direction, it is reasonable for them to envision sustainable growth over the next 10 to xx years.
Do Adjuvants Need To Be Registered In Brazil,
Source: https://www.agribusinessglobal.com/agrochemicals/unpredictability-rules-brazilian-pesticide-registrations/
Posted by: fontanaalmyconver.blogspot.com


0 Response to "Do Adjuvants Need To Be Registered In Brazil"
Post a Comment